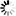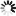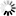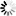4-(1H-Tetrazol-5-yl)benzoic+acid
Catalog Number:
(10800-602)
Supplier:
Rockland Immunochemical
Description:
Catenin α is a novel actin-binding and -bundling protein. Catenin α is responsible for organizing and tethering actin filaments at the zones of E-cadherin-mediated cell-cell contact (1). Monomeric Catenin α can bind strongly to E-Cadherin-β-Catenin, whereas the dimer preferentially binds actin filaments. Different molecular conformations are associated with these different binding states, indicating that Catenin α is an allosteric protein. Catenin α directly regulates actin-filament organization by suppressing Arp2/3-mediated actin polymerization, likely by competing with the Arp2/3 complex for binding to actin filaments (2). Catenin α Protein is ideal for investigators involved in Signaling Proteins, Transcription Proteins, Cancer, Cardiovascular Disease, Invasion/Metastasis, Neurobiology, PKA/PKC Pathway, and WNT Signaling research.
Catalog Number:
(76194-216)
Supplier:
Prosci
Description:
This mAb recognizes TGF beta 1, 2 and 3. Three TGFbs have been identified in mammals. TGFb1, TGFb2 and TGFb3 are each synthesized as precursor proteins that are very similar in that each is cleaved to yield a 112 amino acid polypeptide that remains associated with the latent portion of the molecules. Biologically active TGFb requires dimerization of the monomers (usually homodimers) and release of the latent peptide portion. Overall, the mature region of the TGFb3 protein has approximately 80% identity to the mature region of both TGFb1 and TGFb2. However, the NH2 terminals or precursor regions of their molecules share only 27% sequence identity. TGFb's inhibit the growth of epithelial cells and stimulate the growth of mesenchymal cells.
Catalog Number:
(10800-764)
Supplier:
Rockland Immunochemical
Description:
CCAAT enhancer binding proteins (CEBPs) are a family of transcription factors that all contain a highly conserved, basic-leucine zipper domain at the C-terminus that is involved in dimerization and DNA binding. At least six members of the family have been isolated and characterized to date (CEBP alpha to CEBP zeta). CEBPD is a leucine zipper (LZ) DNA-binding protein that regulates gene expression in a variety of tissues including liver, adipose, lung and intestine. CEBPD is an important transcriptional activator in the regulation of genes involved in immune and inflammatory responses and has been reported to possess many tumor suppressor-like properties.
Catalog Number:
(75933-806)
Supplier:
Rockland Immunochemical
Description:
The mammalian Kelch-like 1 (KLHL1) was initially discovered as a homolog to the Drosophila Kelch gene that is highly expressed in several brain tissues. The predicted protein domain structure of KLHL1 is characteristic of a number of proteins that bind actin, form dimers, and often act as actin-organizing proteins. Based on the presence of anti-sense RNA that spans the transcription and translation start sites as well as the first splice site of KLHL1 in brain tissue of individuals suffering from the neurodegenerative disorder spinocerebellar ataxia type 8 (SCA8), it has been suggested that KLHL1 is involved this disease and that regulation of KLHL1 protein may be affected by antisense RNA expression.
Catalog Number:
(10800-558)
Supplier:
Rockland Immunochemical
Description:
Caspases are a family of cysteine proteases that can be divided into the apoptotic and inflammatory caspase subfamilies. Unlike the apoptotic caspases, members of the inflammatory subfamily are generally not involved in cell death but are associated with the immune response to microbial pathogens. The apoptotic subfamily can be further divided into initiator caspases, which are activated in response to death signals, and executioner caspases, which are activated by the initiator caspases and are responsible for cleavage of cellular substrates that ultimately lead to cell death. Caspase-3 is synthesized as an inactive proenzyme that undergoes proteolytic cleavage by caspases 8, 9 and 10 to produce 2 subunits, termed p20 and p11. These subunits dimerize to form the active enzyme. Caspase-3 proteolytically cleaves and activates other proteins such as caspases 6, 7 and 9.
Catalog Number:
(10286-184)
Supplier:
Bioss
Description:
DNA polymerase lambda (pol lambda), also designated DNA polymerase lambda or Pol Beta2, is a low-fidelity polymerase which plays a role in both spontaneous and DNA damage-induced mutagenesis. Encoded by the POLL gene, pol lambda is a member of the DNA polymerase type-X family. Pol lambda extends primer-terminal mispairs opposite nondamaged DNA templates, suggesting that it may assist in extending mismatched base pairs during normal DNA replication. In addition, pol ?may play a role in the mutagenic bypass of T-T dimers. Proliferating cell nuclear antigen (PCNA), a protein essential to DNA replication, interacts with pol lambda and thus influences the ability of pol ?to synthesize DNA.
Catalog Number:
(10246-968)
Supplier:
Bioss
Description:
Midline-2 is a 715 amino acid protein encoded by the human gene MID2. Midline-2 belongs to the TRIM/RBCC family and contains two B box-type zinc fingers, one B30.2/SPRY domain, one COS domain, one fibronectin type-III domain and one RING-type zinc finger. Midline-2 is a cytoplasmic protein found as a homodimer or heterodimer with Midline-1. It also interacts with IGBP1 (Lymphocyte signaling protein A4). Dimerization is mediated by the tripartite motif (RBCC; RING- and B box-type zinc fingers and coiled coil domains) and microtubule association is dependent on the C-terminal B30.2 domain. Midline-2 is expressed at low levels in fetal kidney and lung, and in adult prostate, ovary and small intestine.
Catalog Number:
(10303-144)
Supplier:
Bioss
Description:
Zinc-finger proteins contain DNA-binding domains and have a wide variety of functions, most of which encompass some form of transcriptional activation or repression. The majority of zinc-finger proteins contain a Krüppel-type DNA binding domain and a KRAB domain, which is thought to interact with KAP1, thereby recruiting histone modifying proteins. Zinc finger and BTB domain-containing protein 5 (ZBTB5) is a 677 amino acid member of the Krüppel C2H2-type zinc-finger protein family. Localized to the nucleus, ZBTB5 contains a BTB domain, also known as a POZ domain, which inhibits DNA binding and mediates homotypic and heterotypic dimerization. Characteristics of the BTB domain suggest that ZBTB5 functions as a transcription regulator.
Catalog Number:
(10303-142)
Supplier:
Bioss
Description:
Zinc-finger proteins contain DNA-binding domains and have a wide variety of functions, most of which encompass some form of transcriptional activation or repression. The majority of zinc-finger proteins contain a Krüppel-type DNA binding domain and a KRAB domain, which is thought to interact with KAP1, thereby recruiting histone modifying proteins. Zinc finger and BTB domain-containing protein 5 (ZBTB5) is a 677 amino acid member of the Krüppel C2H2-type zinc-finger protein family. Localized to the nucleus, ZBTB5 contains a BTB domain, also known as a POZ domain, which inhibits DNA binding and mediates homotypic and heterotypic dimerization. Characteristics of the BTB domain suggest that ZBTB5 functions as a transcription regulator.
Catalog Number:
(10799-446)
Supplier:
Rockland Immunochemical
Description:
14-3-3α/β is a member of the highly conserved 14-3-3 family of proteins which mediate signal transduction by binding to phosphoserine-containing proteins (1). 14-3-3α/β protein has been shown to interact with RAF1 and CDC25 phosphatases, suggesting that it may play a role in linking mitogenic signaling and the cell cycle machinery. 14-3-3-β interacts with the TSC1-TSC2 dimer (2). The interaction required phosphorylation of TSC2 at Ser1210. Binding of 14-3-3-β to TSC2 did not alter the interaction between TSC1 and TSC2, but it reduced the ability of the complex to inhibit phosphorylation of ribosomal protein S6 kinase impairing the ability of the complex to inhibit cell growth. 14-3-3 alpha/beta Protein is ideal for investigators involved in Cell Stress & Chaperone Proteins, Cell Signaling, Cancer research, Cell Cycle, Cellular Stress, Neurobiology, and WNT Signaling research.
Catalog Number:
(BDH7317-1)
Catalog Number:
(10400-408)
Supplier:
Bioss
Description:
Alpha 1 Fetoprotein is a major plasma protein produced by the yolk sac and the liver during fetal life. Alpha fetoprotein expression in adults is often associated with hepatoma or teratoma. However, hereditary persistance of alpha-fetoprotein may also be found in individuals with no obvious pathology. The protein is thought to be the fetal counterpart of serum albumin, and the alpha fetoprotein and albumin genes are present in tandem in the same transcriptional orientation on chromosome 4. Alpha fetoprotein is found in monomeric as well as dimeric and trimeric forms, and binds copper, nickel, fatty acids and bilirubin. The level of alpha fetoprotein in amniotic fluid is used to measure renal loss of protein to screen for spina bifida and anencephaly. Expression has been documented in human adrenal, liver, ovary, testis, and pancreas. ESTs have been isolated from normal human brain, liver/spleen, embryo and uterus tissue libraries.
Catalog Number:
(10400-398)
Supplier:
Bioss
Description:
Alpha 1 Fetoprotein is a major plasma protein produced by the yolk sac and the liver during fetal life. Alpha fetoprotein expression in adults is often associated with hepatoma or teratoma. However, hereditary persistance of alpha-fetoprotein may also be found in individuals with no obvious pathology. The protein is thought to be the fetal counterpart of serum albumin, and the alpha fetoprotein and albumin genes are present in tandem in the same transcriptional orientation on chromosome 4. Alpha fetoprotein is found in monomeric as well as dimeric and trimeric forms, and binds copper, nickel, fatty acids and bilirubin. The level of alpha fetoprotein in amniotic fluid is used to measure renal loss of protein to screen for spina bifida and anencephaly. Expression has been documented in human adrenal, liver, ovary, testis, and pancreas. ESTs have been isolated from normal human brain, liver/spleen, embryo and uterus tissue libraries.
Catalog Number:
(103680-614)
Supplier:
Sino Biological
Description:
A DNA sequence encoding the native human S100A11 (NP_005611.1) (Met 1-Thr 105) was expressed.
Catalog Number:
(76109-352)
Supplier:
Bioss
Description:
Hemostasis following tissue injury involves the deployment of essential plasma procoagulants (Prothrombin and Factors X, IX, V and VIII), which are involved in a blood coagulation cascade that leads to the formation of insoluble Fibrin clots and the promotion of platelet aggregation. Coagulation Factor X (Stuart Prower factor, FX, F10) is a vitamin K-dependent, single chain serine protease that is synthesized in the liver and circulates as an inactive precursor. The mature form of Factor X (Factor X A) is generated by Factor IX A- or Factor VII A-mediated cleavage at the tripeptide sequence, Arg-Lys-Arg, to yield a disulfide linked dimer. Together with the cofactor Factor V A and Ca²⁺ on the surface of platelets or endothelial cells, Factor X A coordinates as part of the prothrombinase complex, which mediates proteolysis of Prothrombin into active Thrombin. Mutations at the Factor X locus resulting in Factor X deficiencies can contribute to hemorrhagic diathesis.
Inquire for Price
Stock for this item is limited, but may be available in a warehouse close to you. Please make sure that you are logged in to the site so that available stock can be displayed. If the
Stock for this item is limited, but may be available in a warehouse close to you. Please make sure that you are logged in to the site so that available stock can be displayed. If the
This product is marked as restricted and can only be purchased by approved Shipping Accounts. If you need further assistance, email VWR Regulatory Department at Regulatory_Affairs@vwr.com
-Additional Documentation May be needed to purchase this item. A VWR representative will contact you if needed.
This product has been blocked by your organization. Please contact your purchasing department for more information.
The original product is no longer available. The replacement shown is available.
This product is no longer available. Alternatives may be available by searching with the VWR Catalog Number listed above. If you need further assistance, please call VWR Customer Service at 1-800-932-5000.
|
|||||||||